What Is The Molecular Geometry Of So32−?
What Is The Molecular Geometry Of So32−?. It shows the bonding between the atoms of a. The electron geometry of so2 is formed in the shape of a trigonal planner.
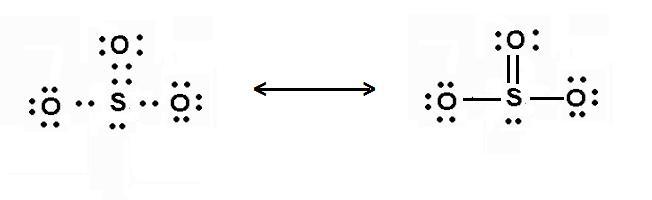
What is the molecular shape of so3 − 2? What is the molecular shape of so3 − 2? The electron geometry of so2 is formed in the shape of a trigonal planner.
As The One Pair Remained.
Trigonal pyramidal 2−central atom:stotal vsep:73 x double bonds− 3 pairsrevised total:4geometry:trigonal pyramidal (based on tetrahedral)4. So, oxygen and sulfur atoms have six electrons in their. There is a lone pair of electrons present on the central sulfur atom in.
It Is A Polar Molecule Because It Has Dipole Moments Due To Its Trigonal Pyramidal Geometry.
What is the molecular shape of so3 − 2? What is the molecular geometry of so32−? Which of the following describes a scenario in which the molecular geometry would have the smallest bond angle (s)?
What Is The Molecular Shape Of So3 − 2?
The lewis structure for potassium has 1 valence electron, while the lewis structure for monoatomic chlorine has 7 valence electron. Typesp2bond angle120ogeometrytrigonal planar what is the shape of so3 2?2−central atom:stotal vsep:73 x double bonds− 3 pairsrevised total:4geometry:trigonal pyramidal. What is the molecular geometry of of 2?
What Is The Molecular Geometry Of So32−?
The molecular geometry of s o 3 2 − is a trigonal pyramidal structure with bond angles of 1 0 7. Both sulfur and oxygen are located at via group in the periodic table. How many lewis structures does so3?
Note That The Precise Angle Is 106.0.
The geometry of the so3 molecule ion can then be predicted using the valence shell electron pair repulsion theory (vsepr theory) and molecular hybridization theory, which states that. Trigonal pyramidal 2−central atom:stotal vsep:73 x double bonds− 3 pairsrevised total:4geometry:trigonal pyramidal (based on tetrahedral)4. It shows the bonding between the atoms of a.
Post a Comment for "What Is The Molecular Geometry Of So32−?"